Overconsumption of sugar fuels low-grade systemic inflammation and microbial imbalance, accelerating periodontal inflammation and systemic diseases. Limiting sugar could be key to better oral and overall health.
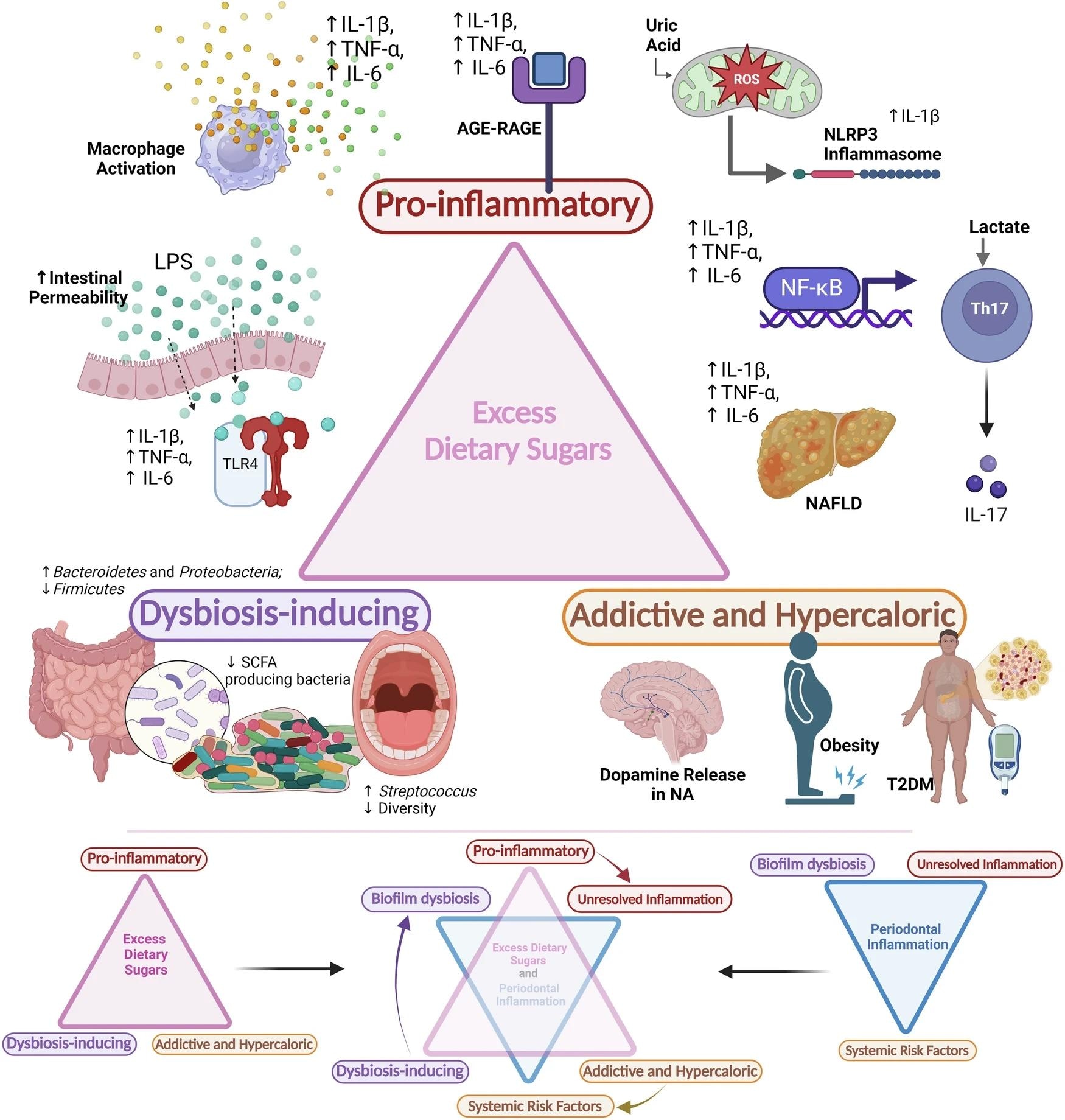
Excess dietary sugars are potentially pro-inflammatory, dysbiosis-inducing, addictive, and hypercaloric and can initiate and aggravate periodontal inflammation. (AGE- advanced glycation end-products; RAGE- receptor for advanced glycation end-products; ROS- reactive oxygen species; LPS- lipopolysaccharide; TLR-4- toll-like receptor-4; NAFLD- Non-alcoholic fatty liver disease; NF-κB- nuclear factor kappa-B; SCFA- short-chain fatty acids; NA- Nucleus accumbens; T2DM- type-2 diabetes mellitus; IL- interleukin; TNF- α- tumour necrosis factor-α; NLRP3- NOD-, LRR- and pyrin domain-containing protein 3) ( Created in BioRender. Biorender, P. (2024) BioRender.com/h79u248).
In a review published in the journal BDJ Open, researchers from India discussed the health effects of excessive sugar consumption and its potential role in periodontal inflammation. They found that excessive dietary sugars, particularly fructose and sucrose, can cause systemic inflammation, trigger gut barrier dysfunction and endotoxemia, and induce dysbiosis in gut and oral microbiota. These effects contribute to obesity, metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), and other systemic conditions, which are risk factors for periodontal inflammation.
Background
Excessive sugar intake is also linked to a potential addiction-like response due to its impact on the brain’s reward system, similar to that seen with drugs such as cocaine. Sugar triggers dopamine release in the brain’s pleasure centers and activates the endogenous opioid system, potentially leading to addictive behaviors such as bingeing, craving, and withdrawal. Glucose and fructose are integral to many modern foods, leading to overconsumption and associated health risks like systemic inflammation, gut dysbiosis, and metabolic syndrome. Oral diseases, affecting 3.5 billion people globally, share sugar as a major risk factor. The American Heart Association and World Health Organization recommend limiting sugar intake to reduce these risks. Despite its critical role, the impact of dietary sugars on periodontal inflammation has been underexplored. Therefore, researchers in the present literature review explored the effects of excess dietary sugars, particularly fructose and sucrose, on periodontal inflammation.
Sugar and inflammation
Excess dietary sugar contributes to low-grade systemic inflammation, gut barrier dysfunction, and immune dysregulation, all of which are linked to various health conditions, including periodontal inflammation. Fructose consumption, in particular, disrupts tight junction proteins in the intestinal barrier, increasing permeability and allowing bacterial endotoxins to enter the bloodstream. This process, known as endotoxemia, exacerbates systemic inflammation and contributes to the development of NAFLD, which further fuels the inflammatory cycle. NAFLD then perpetuates systemic inflammation, which is linked to diseases such as metabolic syndrome and cardiovascular disease.
Sugar-rich diets also directly affect immune function, promoting the production of pro-inflammatory cytokines and oxidative stress, further exacerbating inflammation. This chronic inflammation has a bidirectional relationship with periodontitis. Studies show that excessive intake of sugar and sugar-sweetened beverages is associated with a higher risk of periodontal disease, particularly in adolescents. High sugar intake is linked to obesity and metabolic syndrome, which are associated with various NCDs, including type 2 diabetes, cardiovascular disease, and cancer. These conditions also have a bidirectional relationship with periodontal inflammation. Moreover, the pro-inflammatory state triggered by NAFLD and obesity exacerbates periodontal inflammation through systemic pathways, including the production of pro-inflammatory cytokines such as TNF-α and IL-6. Future studies using genomic technologies could provide deeper insights into sugar’s impact on gene expression related to inflammation. Nutrigenomics, in particular, may help explore how dietary sugars influence gene expression patterns related to periodontal inflammation and systemic disease progression.
Sugar-induced microbial dysbiosis
The human microbiome, comprising all microbes in the body and their genomic content, plays a vital role in health and disease. Dysbiosis, the disruption of the microbiota’s symbiotic balance, can result from various factors, including diet and lifestyle.
The gut microbiota mainly consists of Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia. A high-sugar diet is linked to gut dysbiosis, characterized by increased Proteobacteria and Bacteroidetes and decreased Firmicutes and short-chain fatty acid (SCFA) producers, leading to increased intestinal permeability and inflammation. Fructose and sucrose, in particular, have been shown to promote dysbiosis, leading to increased levels of harmful gram-negative bacteria and decreased production of beneficial SCFAs, such as butyrate. This promotes gut barrier dysfunction and systemic inflammation. Studies in rats and humans show that high fructose and sucrose consumption alters the gut microbiota’s composition, decreases SCFA production, and promotes inflammation and lipid accumulation in the liver. Notably, fructose from whole fruits does not negatively impact gut health.
The oral microbiome, containing around 700 bacterial species, is also affected by excessive sugar intake. Short-term sucrose rinses and in vitro studies indicate that high sugar levels can reduce microbial diversity and increase harmful bacteria like Actinomyces and Streptococcus. This dysbiosis in the oral microbiome contributes directly to periodontal inflammation by increasing the abundance of pathogenic species, reducing diversity, and promoting the formation of biofilms that exacerbate gingival inflammation. A systematic review suggests that high sugar consumption significantly contributes to oral dysbiosis, but further research is needed to understand its full microbiological impact.
Clinical relevance
Three factors contribute to periodontal inflammation: dysbiosis of oral biofilms, an unresolved inflammatory response leading to tissue damage, and systemic conditions that worsen inflammation. Current strategies for preventing and managing periodontal inflammation mainly focus on controlling biofilms through brushing and professional cleanings. However, a multidimensional approach addressing lifestyle factors is needed. Dietary interventions have shown that a micronutrient-rich, low-sugar diet can significantly reduce gingival inflammation and the presence of pathogens.
Conclusion
In conclusion, the current evidence suggests that excessive sugar consumption may promote inflammation, induce dysbiosis, and contribute to systemic risk factors for periodontal inflammation. Despite limited research, sugar is considered a modifiable lifestyle risk factor for periodontal disease. As genomic technologies advance, future research could explore the molecular mechanisms by which dietary sugars influence inflammation at the gene expression level. Dietary interventions that limit sugar and ultra-processed food intake may benefit periodontal health and warrant further investigation. Promoting moderation in sugar consumption could serve as a practical, cost-effective public health strategy to prevent and manage periodontal inflammation.
Source link : News-Medica