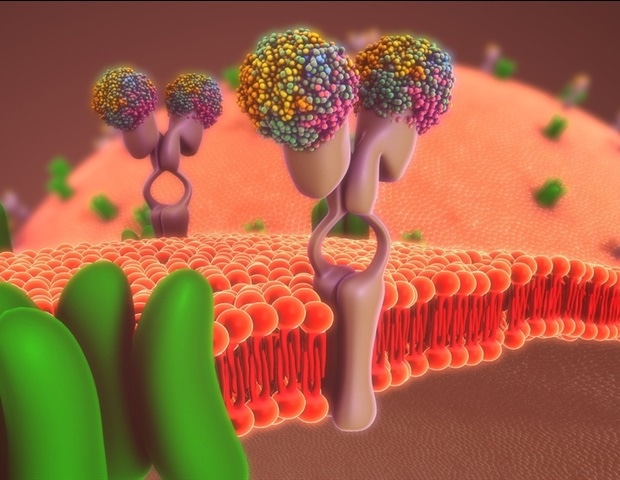
Researchers at the Paul Scherrer Institute PSI have succeeded in elucidating the structure of specific photoreceptors. With their help, it may be possible to switch cellular activities on and off using light. This capability could become an important tool in biological research and medical applications.
Researchers in biology and medicine have long dreamed of controlling the activities of cells without, for example, having to use chemicals. After all, in a structure as complex as an entire organism, unwanted side-effects can often arise. The ideal solution would therefore be a type of remote control for cells, which would allow the functions of individual organs to be better examined and understood, and could even be used for therapeutic purposes. Remote control using light would be ideal for this, as it would enable organs and tissues deep inside the body to be influenced in a very selective and non-invasive way. However, such a process also requires a cellular light receiver in the relevant organs. The receptors that receive light impulses in the retina of our eyes – called rhodopsins – could be suitable for this. With such photoreceptors, it might be possible to switch certain cell functions on and off using a light impulse. This would work more rapidly and in a more targeted manner than drugs, which take a long time to take effect and often have unwanted side-effects because they cannot simply be activated in just one specific organ.
In the neurosciences, something similar is already working and is currently being tested in animal models to investigate brain diseases such as Parkinson’s and epilepsy: Light-controlled ion channels from single-celled organisms are being incorporated into neurons using genetic engineering. In the animal model, these ion channels in the cell membrane open when exposed to blue light, for example, and allow positively charged ions to flow into the neuron. In a chain reaction, further channels open, creating an electrical signal – the neuron becomes active.
A new kind of optogenetics
But such light-controlled ion channels only work in nerve cells. The goal of this research, however, is to stimulate other cells and organs in the organism to control a variety of bodily functions. For example, one could investigate the heart’s natural pacemaker, or the mechanisms of chronic pain, anxiety, depression, and other mental illnesses. It might be possible to develop effective cell therapies for hormonal malfunctions as well as immune, heart, and other diseases, including cancer.
To this end, researchers led by Gebhard Schertler of the PSI Center for Life Sciences are working on a new kind of optogenetics. In this approach, it is light receptors similar to the rhodopsins in our retina that become active: Triggered by a light pulse, they couple to proteins in the cell and thus initiate specific cellular signalling processes that take place in all organs. The PSI researchers have joined forces with leading colleagues in Germany and England; together they were awarded a coveted ERC grant: funding of nearly eight million euros from the European Research Council. Their project, Switchable rhodOpsins in Life Sciences (SOL), has three goals: 1. Find rhodopsins that can do this and elucidate their structure to better understand how they work. 2. Modify such rhodpsins, using molecular biological methods, to optimise them for switching processes in various bodily functions. 3. Use the switches to better understand the signalling mechanisms of the proteins; use them as a tool in research and, on that basis, develop gene therapeutics.
The structural elucidation of proteins is a core competence of PSI, thanks to its high-resolution large research facilities. And PSI researchers have now made two significant steps towards SOL’s first goal, as they report in two new studies: First, they succeeded in finding a suitable rhodopsin and modifying it in such a way that it remains stable in the active state and thus can be examined. And second, the structure of this active state was clarified using a cryo-electron microscope at ETH Zurich.
A switch that bends and stretches
Rhodopsins are proteins. They are among the most important photoreceptors in the animal world. They have an elongated molecule in the middle, called retinal, that is derived from vitamin A. When a light pulse hits this molecule, it absorbs the energy and changes its shape within a quadrillionth of a second. A curved molecule – called the 11-cis form – becomes an elongated one – called the all-trans form. Through this transformation, the retinal also changes the structure of the entire rhodopsin so that it now can bind to other proteins in the cell membrane, so-called G proteins. Therefore, these light-sensitive rhodopsins also belong to the GPCR (G protein-coupled receptor) family, as rhodopsin-G protein complexes stimulate other proteins to react, triggering a whole series of biochemical processes leading, for example, to the transmission of a visual signal to the brain.
The human body possesses hundreds of different types of GPCRs, which are located in the cell membranes, receive signals from the outside, and pass them along to the inside of the cell. In this way, they control many bodily functions. That’s why roughly 40 percent of all medications target GPCRs with active ingredients that dock onto their receptors.
The advantage of simple photoreceptors
Rhodopsins are found in the retina of the human eye. In the rod cells, for example, they are responsible for distinguishing between light and dark at night. However, like those of most vertebrates, these rhodopsins are monostable. This means that once the retinal has changed by light, it leaves the protein and has to be regenerated. Only then is it available for the next switching process. This is too complicated to allow this molecule to be used effectively as an optogenetic switch, since enzymes would also have to be used to regenerate it.
Many invertebrates, such as squid, insects, and spiders, have bistable rhodopsins. «From an evolutionary perspective, these are actually a more primordial form of rhodopsins, and less sensitive,» says Gebhard Schertler. They offer advantages for optogenetics, however: The retinal remains in the protein after being switched on, and with a second light pulse it can immediately return to its original form and switch the cellular process off again.
The rhodopsin of a jumping spider species, for example, proved to be robust and easy to produce, unlike other bistable rhodopsins. This qualified it as a possible optogenetic switch.
With the Swiss Light Source SLS at PSI, it was possible to determine the molecular structure of this spider rhodopsin in its inactive ground state. But before it could be used as an optogenetic switch, its structure in the active form also had to be known precisely. This state, however, when the retinal is stretched and the rhodopsin binds to the G protein, is extremely short-lived.
How to make proteins happy
In one study, which recently appeared in the journal PNAS, lead author Matthew Rodrigues now reports how they managed to stabilise the active state to be able to elucidate its structure: by making a tiny modification to the retinal. «The properties of the retinal remain the same, but the modification – one small additional molecular ring – ensures that it apparently fits better into the binding pocket of the protein,» reports Rodrigues. «It stays there for hours. As we structural biologists say, it’s happy.» Now the conditions were in place to examine the structure of the active rhodopsin in conjunction with a G protein.
A mixed protein
In a second study, now published in Nature Communications, first author Oliver Tejero and last author Ching-Ju Tsai did exactly that. «However, as expected, it was found that a spider protein (rhodopsin) naturally never fits optimally with a human protein (the G protein),» says Tsai. «So we compared spider G proteins with those of humans and assembled a chimera from both forms.» The researchers replaced the end part of the gene sequence of the human protein, which contains the code for the docking site, with that of the spider.
With additional genetic modifications in the actual light receptor, they addressed another problem: The spider rhodopsins are both activated and deactivated by light of the same wavelength. «This means that a light pulse produces a hopeless hodgepodge of activated and deactivated states in a cell sample,» says Tsai. Naturally, this is bad for a switch that is intended to turn on or off in a targeted manner. «With our modifications, we have ensured that switching on and off now takes place with different colours of light.»
However, such «colour tuning» by means of genetic engineering is only just beginning. The next step in the fundamental research into these new optogenetic switches will now be to find out how the proteins involved need to be designed to enable control using other colours of light. This would then make it possible to selectively switch different cell functions on or off. It is also a matter of constructing the switches so that they are not only sensitive to blue, orange, and green light, but also, for example, to infrared light. «The big question remains, if optogenetics is actually to be used in everyday medical practice, how the light will get to the rhodopsin,» says Matthew Rodrigues. «You could implant the light source into the body. But the much more elegant and gentler method would be to work with infrared light. This can penetrate body tissue.»
The largest part of the protein engineering, project leader Gebhard Schertler confirms, is still to come, now that the structural basics are known. Ultimately, the goal is to put together a whole assembly kit of light-activated GPCRs that can be used for various purposes in the organism.
- Tejero, O., et al. (2024). Active state structures of a bistable visual opsin bound to G proteins. Nature Communications. doi.org/10.1038/s41467-024-53208-2.
- Rodrigues, M. J., et al. (2024). Activating an invertebrate bistable opsin with the all-trans 6.11 retinal analog. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2406814121.
Source link : News-Medica